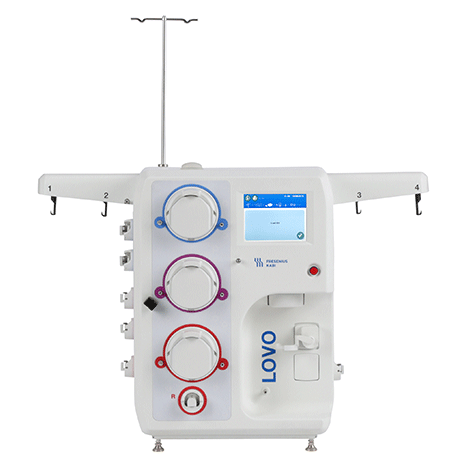
Only Lovo delivers automated, functionally closed cell processing through spinning membrane filtration.
See how Lovo brings greater efficiency and consistency to your lab. >
Interested in learning more about our other Cell Processing System?
For Laboratory Use Only
Designed with you in mind.
Hear the people who designed Lovo talk about how its technology, design, and performance were driven by the needs of labs like yours.
Each cell therapy manufacturing process is unique. Lovo's flexibility supports multiple applications, at any stage of your operation.
Whether you're working with millions or billions of cells, in 10mL or 22L, Lovo adapts with you to easily scale your process, from Phase 1 through Commercialization.
You don't need to trade cell recovery with washout efficiency. Lovo’s spinning membrane filtration allows you to process a wide range of cell volumes and concentrations quickly, while maximizing recovery and cell viability.
DXT Data Management System for use with the Lovo Cell Processing System, is an open architecture software platform supported by our team of networking specialists. Lovo and DXT support 21 CFR Part 11 compliance.
Stay Informed.
Sign up below to receive the latest Lovo news and updates.
See Us In Action.
Come see what Lovo is all about. We’ll walk you through the features and arrange for a demonstration.
Advanced Therapies Europe (ATE)
September 2-4, 2025
Barcelona, Spain
CAR-TCR Summit
September 23-26, 2025
Boston, MA
Meeting on the Mesa
October 6-8, 2025
Phoenix, AZ
Document
The Lovo Cell Processing System is for laboratory use only and may not be used for direct transfusion. Appropriate regulatory clearance is required by the user for clinical use.
For applications requiring regulatory clearance or approval, Users may request required Lovo technical documentation from Fresenius Kabi to support their submission.
Refer to the Lovo Cell Processing System Operator’s Manual, DXT Administrator’s Guide and DXT User’s Guide for a complete list of warnings and precautions associated with the use these products.
References
1-4Data on File at Fresenius Kabi USA
About Fresenius Kabi USA
Fresenius Kabi is a global health care company that specializes in lifesaving medicines and technologies for infusion, transfusion and clinical nutrition.
Our products are used to help care for critically and chronically ill patients. The people of Fresenius Kabi are driven by a common purpose to put lifesaving medicines and technologies in the hands of people who care for patients, and to find answers to the challenges they face.