Lovo works with
any manufacturing process.
Lovo can automate preparation for magnetic selections, simplifying and expediting day 0 processing. With Lovo 3.0 software, you have the option to incorporate mid-procedure, timed incubations in your protocol design.
| Measure | Result | |
|---|---|---|
| Source Products | Collections | n=5 |
| Volume | 163.3 ± 28.5 mL | |
| Total Nucleated Cells (TNC) | 10.9 ± 3.4 x 109 | |
| Platelet Count | 4.46 ± 0.92 x 10 11 | |
| Processing Time | Automated Processing Time (With incubation) | 58 minutes |
| Max Processing Time | 61 minutes | |
Source: LOVO 2.X Blood Run Protocol Report: 223-DER-048957 – Data on file at Fresenius Kabi USA.
| Measure | Result | |
|---|---|---|
| 2 wash cycles to remove platelets and prepare for incubation | Platelet Depletion | 98.4 ± 1.0% |
| Targeted Pre-Incubation Volume | 100 mL | |
| Actual Pre-Incubation Volume | 100.6 ± 1.2 mL | |
| Full Procedure | TNC recovery | 97.2 ± 3.7% |
| TNC Viability | 96.3 ± 1.4% | |
Source: LOVO 2.X Blood Run Protocol Report: 223-DER-048957 – Data on file at Fresenius Kabi USA.
Quickly and easily remove platelets from leukapheresis products while recovering TNCs and maintaining cell viability.
| Measure | Result | |
|---|---|---|
| Collections (5L) |
Fenwal Amicus | n=6 |
| Spectra Optia | n=8 | |
| Source | Volume | 90.8 ± 10.2 ml |
| TNC | 4.7 ± 1.8 x 109 | |
| Final Product |
TNC Recovery | 98.6% |
| TNC Viability | 97.5% | |
| Target Final Volume | 95 mL | |
| Actual Final Volume | 95.3 ± 0.6 mL | |
| Processing Time | Average | 11:01 min |
| Maximum | 11:29 min | |
Source: LOVO 2.X Product Quality Test Results Design Review: 223-DER-048958 – Data on file at Fresenius Kabi USA.
Pre/Post Lovo Wash Platelet Count & Depletion
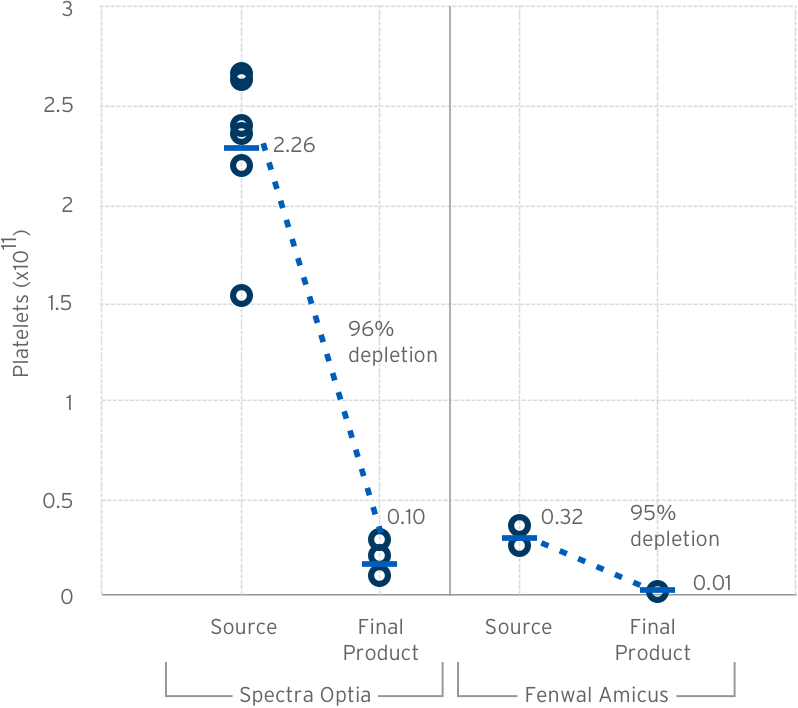
Source: LOVO 2.X Product Quality Test Results Design Review: 223-DER-048958 – Data on file at Fresenius Kabi USA.
Quickly and easily wash and concentrate up to 22L of cultured cells in one automated and functionally closed system.
Study objective
Goals
| Cell Type | Lovo Fresenius Kabi |
CS5+ Haemonetics |
Manual |
|---|---|---|---|
| Recovery (%) | 93 ± 4 | 87 ± 3 | 91 ± 4 |
| Processing Time (min) | 30 ± 1 | 25 ± 4 | 61 ± 11 |
| Washout Efficiency (%) | 99.5 | 99.9 | 99.8 |
| Final Volume (mL) | 150 ± 1 | 153 ± 3 | 151 ± 6 |
| Cellular CQAs1 | comparable | ||
| Automated | ✓ | ± | x |
| Functionally Closed | ✓ | ± | x |
1 Critical Quality Attributes (CQAs) Measured: viability assessment, phenotype, cell functionality, ancillary materials.
Source: Lovo New Membrane Cultured Cell Testing Results: 223-DER-066723 – Data on file at Fresenius Kabi USA.
Wash cryopreserved products and resuspend cells in your preferred buffer or culture media.
| Measure | Lovo 2.0 (three-cycle) |
|---|---|
| Number of Runs | 6 |
| PCV% | 8.4% (6.9 - 11.4) |
| Viable CD34+ Cell Recovery | 84% (61 - 93) |
| CD34+ Cell Viability | 92% (81 - 94) |
| DMSO Elimination | 97% (97 - 98) |
| Total Processing Time | 62 min |
† Data is expressed as median and (IQR)
Source: B.Mfarrej, et al. Pre-clinical assessment of the Lovo device for dimethyl sulfoxide removal and cell concentration in thawed hematopoietic progenitor cell grafts. Cytotherapy, Volume 19, Issue 12, 1501-1508.
3-Cycle Wash1
Post Thaw Recovery
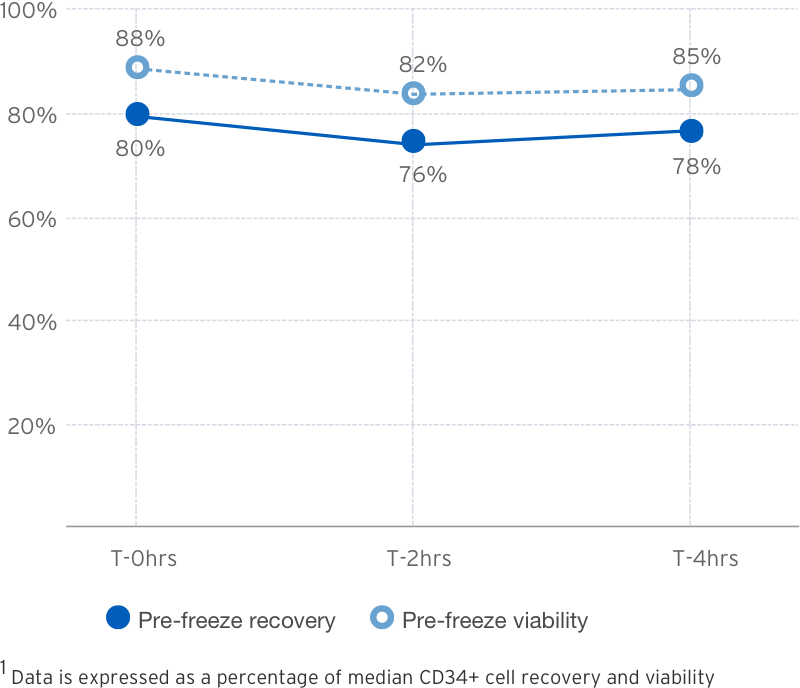
Source: B.Mfarrej, et al. Pre-clinical assessment of the Lovo device for dimethyl sulfoxide removal and cell concentration in thawed hematopoietic progenitor cell grafts. Cytotherapy, Volume 19, Issue 12, 1501-1508.
Stay Informed.
Sign up below to receive the latest Lovo news and updates.
See Us In Action.
Come see what Lovo’s all about. We’ll walk you through the features and arrange a demo.
Advanced Therapies Europe (ATE)
September 2-4, 2025
Barcelona, Spain
CAR-TCR Summit
September 23-26, 2025
Boston, MA
Meeting on the Mesa
October 6-8, 2025
Phoenix, AZ
The Lovo Cell Processing System is for laboratory use only and may not be used for direct transfusion. Appropriate regulatory clearance is required by the user for clinical use.
For applications requiring regulatory clearance or approval, Users may request required Lovo technical documentation from Fresenius Kabi to support their submission.
Refer to the Lovo Cell Processing System Operator’s Manual for a complete list of warnings and precautions associated with the use these products.