Lovo blends flexible, customizable features to meet your current needs and adapt to future requirements.
Lovo's intuitive operation simplifies your processes while minimizing operator interaction. Easily configure parameters and create protocols for different cells, different applications, and different trials — all on the same platform. Then lock down for manufacturing, and scale up as your operation evolves.
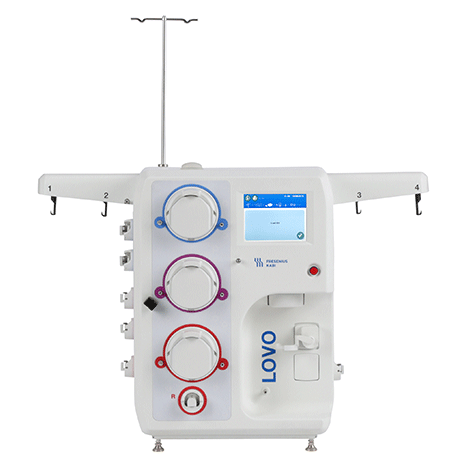

Process a wide range of cell volumes and concentrations with consistent results, every time.
With as little as 3 operator inputs, you can set the parameters for the procedure and let Lovo do the work for you.
Our engineers support researchers on the cutting edge of science through active collaboration — an ongoing dialogue that improves our technology, furthers your objectives, and helps us all reimagine what’s possible.
Simplify manufacturing with functionally closed disposables and ancillaries that provide mid procedure access to your product.
Lovo DXT Data Management is an open-architecture software platform supported by our team of networking specialists. Lovo DXT supports 21 CFR Part 11 compliance.
1 Data Transfer
The LOVO Cell Processing system is for laboratory use only. Unless the user has obtained advance clearance or approval from the appropriate regulatory agency, cells processed on this system are not intended for diagnostic purposes, direct transfusion, or for use in the production of therapeutic products or vaccines for clinical use. For applications requiring regulatory clearance or approval, users may request the required LOVO technical documentation from Fresenius Kabi to support their submissions.
Refer to the LOVO Cell Processing System Operator’s Manual for a complete list of warnings and precautions associated with the use of this device.